OQC Toolkit
Templates to assist research teams with conducting, tracking and/or monitoring research activities.
These documents are available for research teams to download and adapt for their own use.
Template Title |
Version Date |
|---|---|
|
September 2021 |
|
|
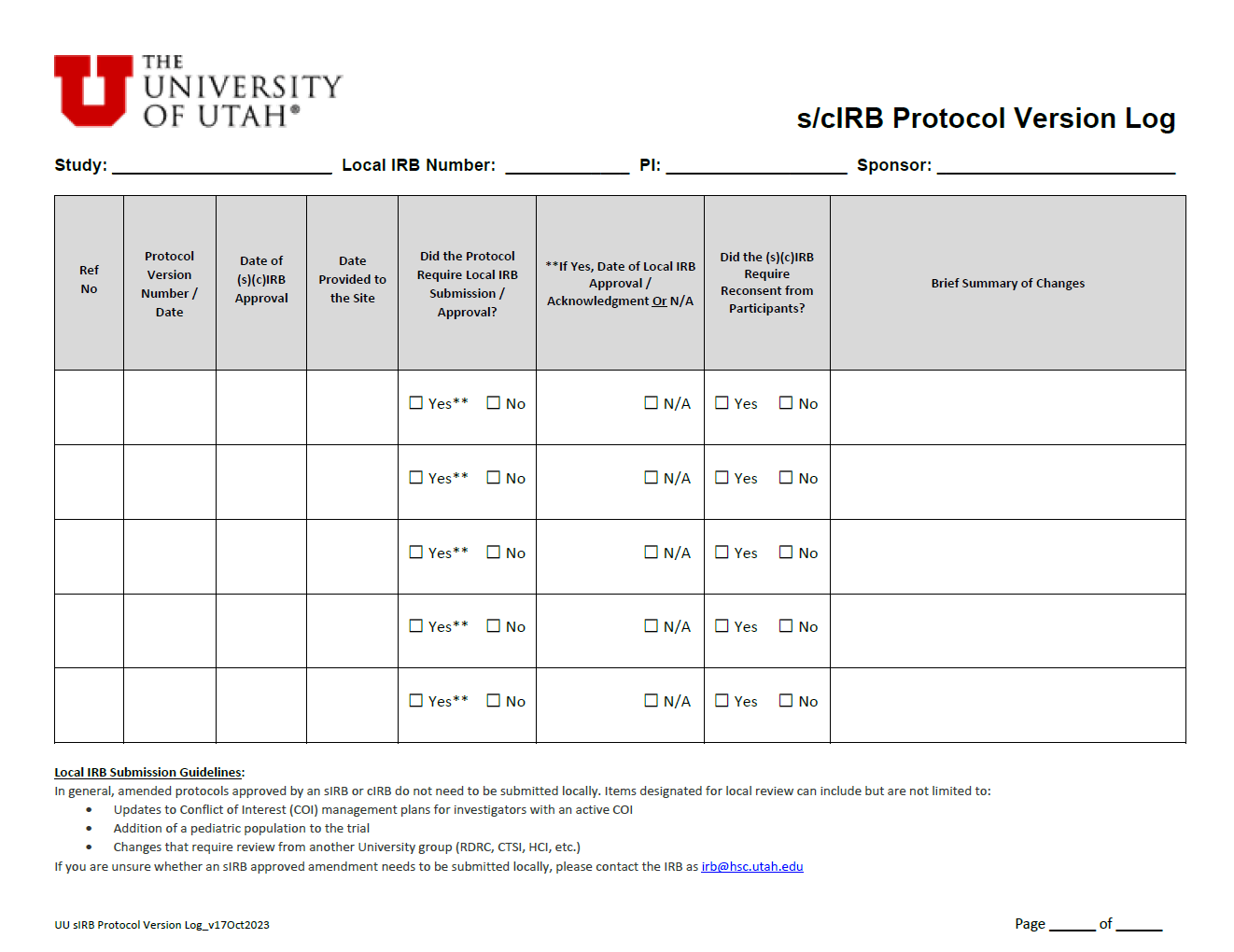
Central IRB Protocol & Consent Version Logs |
October 2023 |
|
June 2020 |
|
|
December 2022 |
|
|
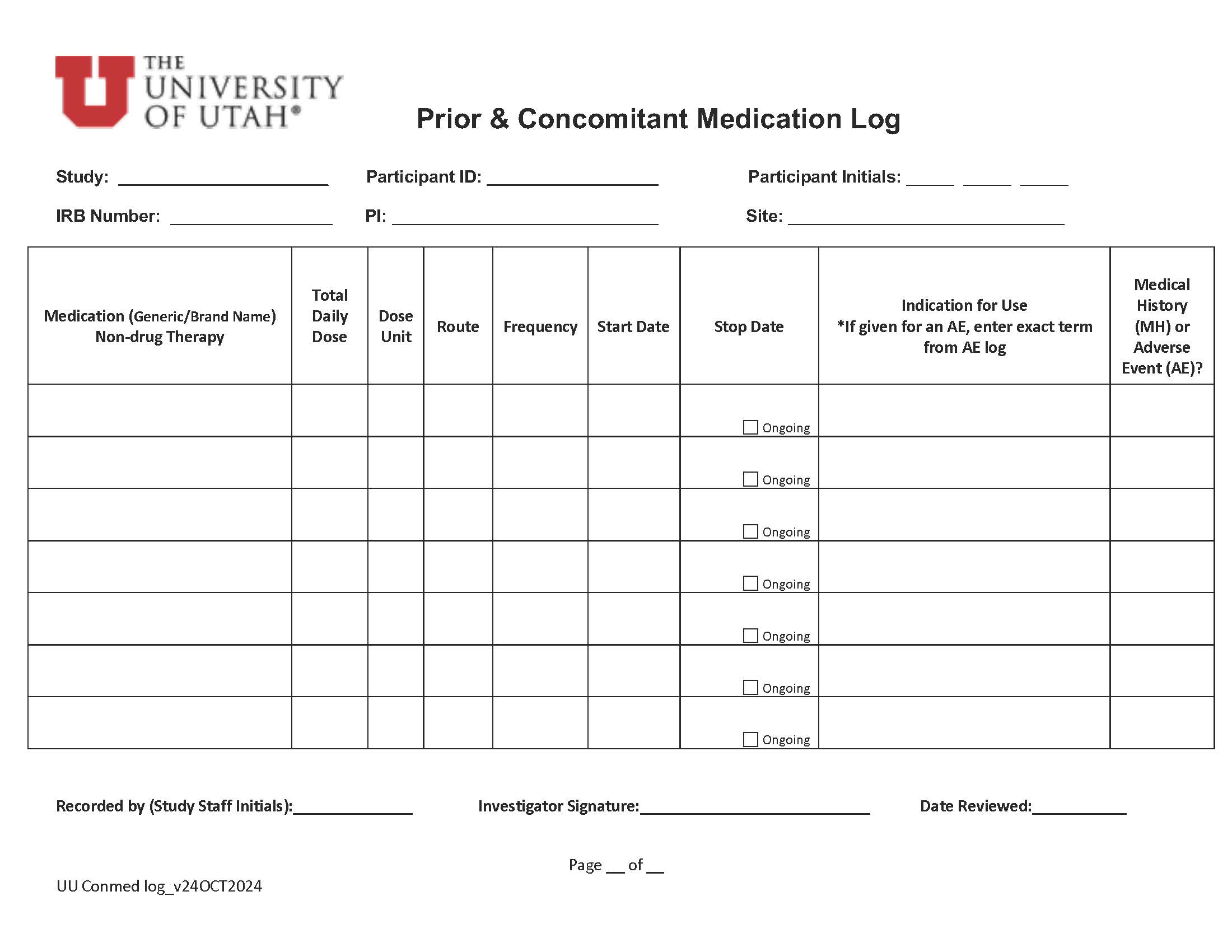
October 2024 |
|
|
June 2020 |
|
|
June 2020 |
|
|
June 2020 |
|
|
April 2024 |
|
|
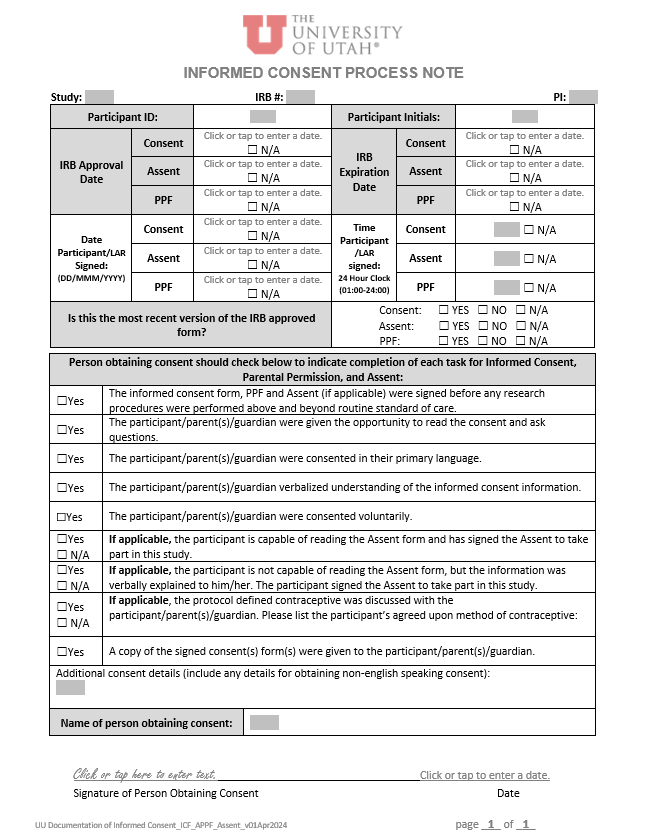
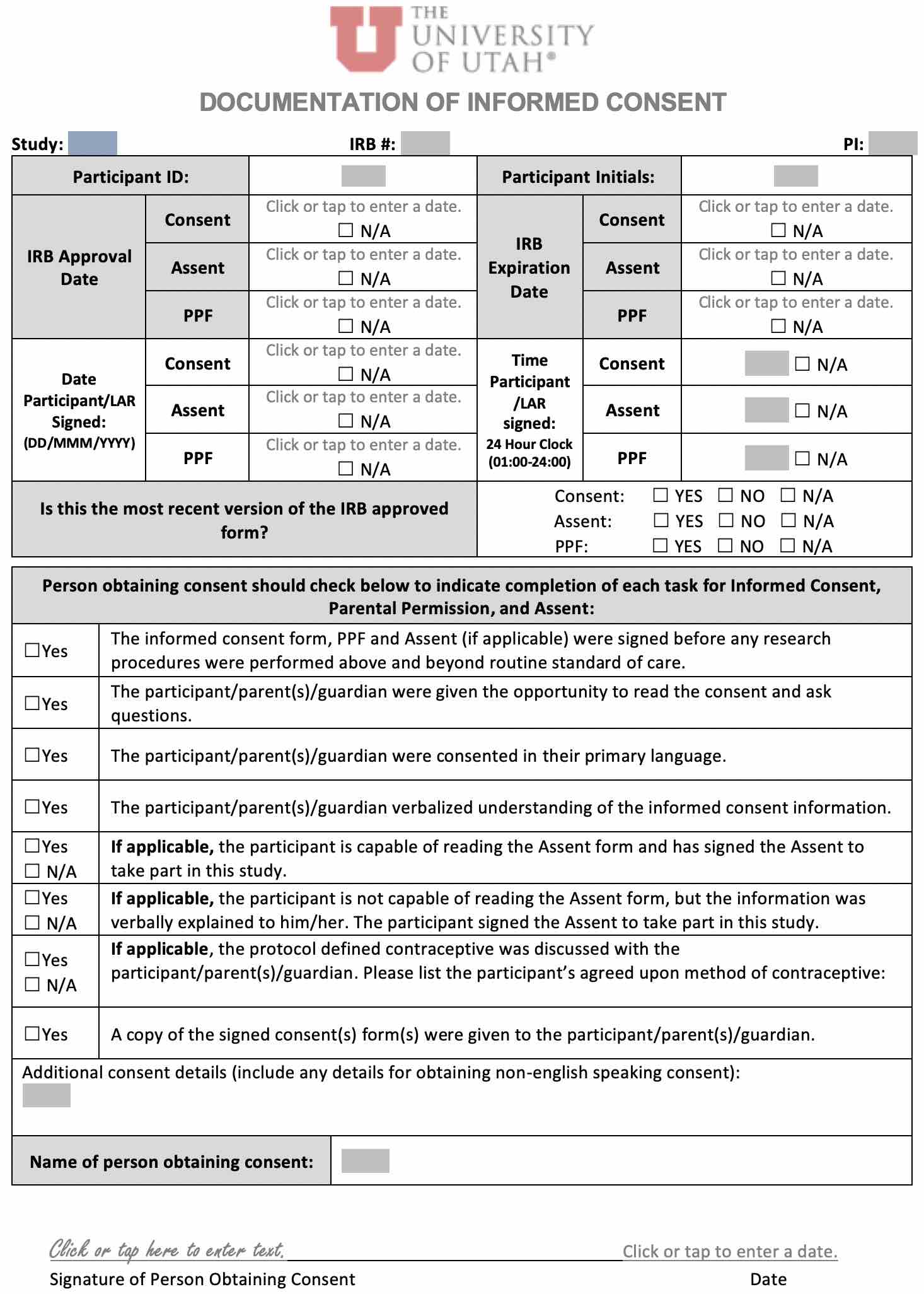
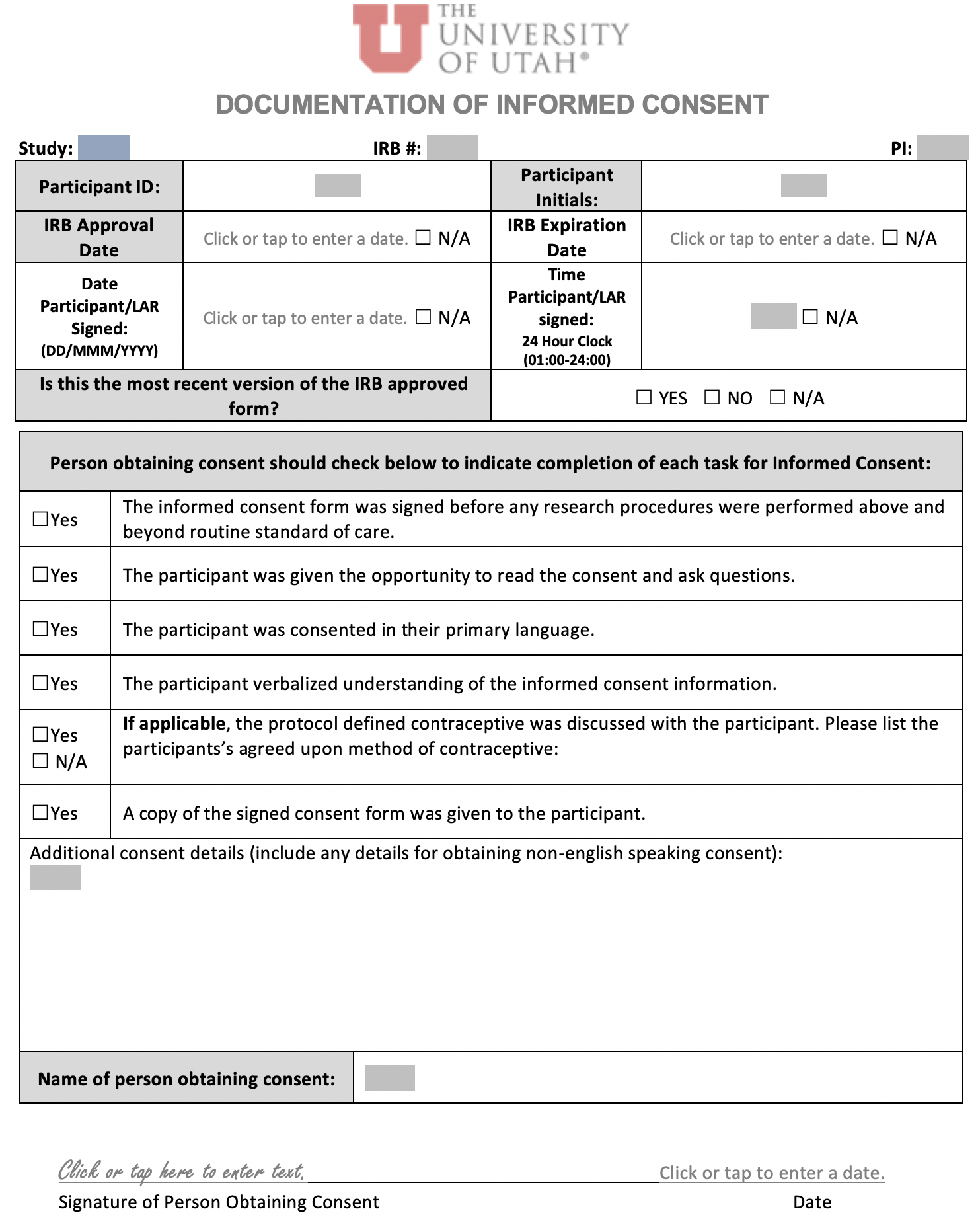
Informed Consent Process Note - Consent, Assent, Parental Permission |
April 2024 |
|
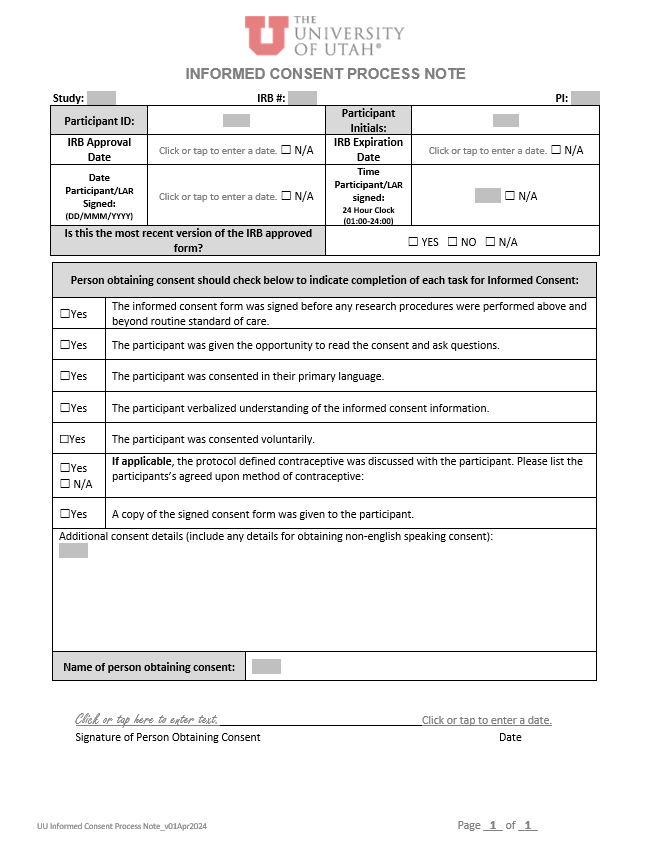
Informed Consent Process Note - Non-English Speaking Participants |
June 2024 |
|
Material Transfer Agreements (MTA) and/or Data Transfer Agreements (DTA) Checklist |
September 2021 |
|
June 2020 |
|
|
June 2020 |
|
|
June 2020 |
|
|
June 2020 |
|
|
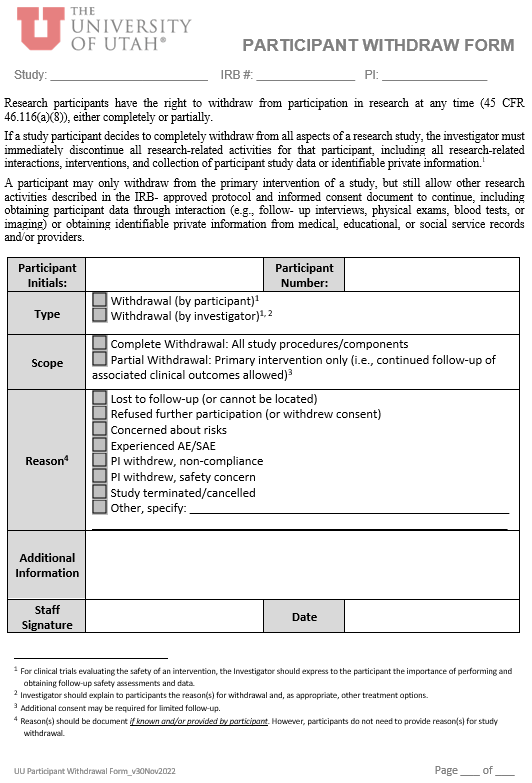
Participant Withdrawal Form Template |
November 2022 |
|
June 2020 |
|
|
June 2020 |
|
|
April 2022 |
|
|
February 2026 |
|
|
June 2020 |
|
|
June 2020 |
|
|
November 2021 |
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview
Preview