UUSOP-2: FDA Inspections
Supplement A - Institutional Assistance for FDA Inspections
Version Date: August 13, 2025
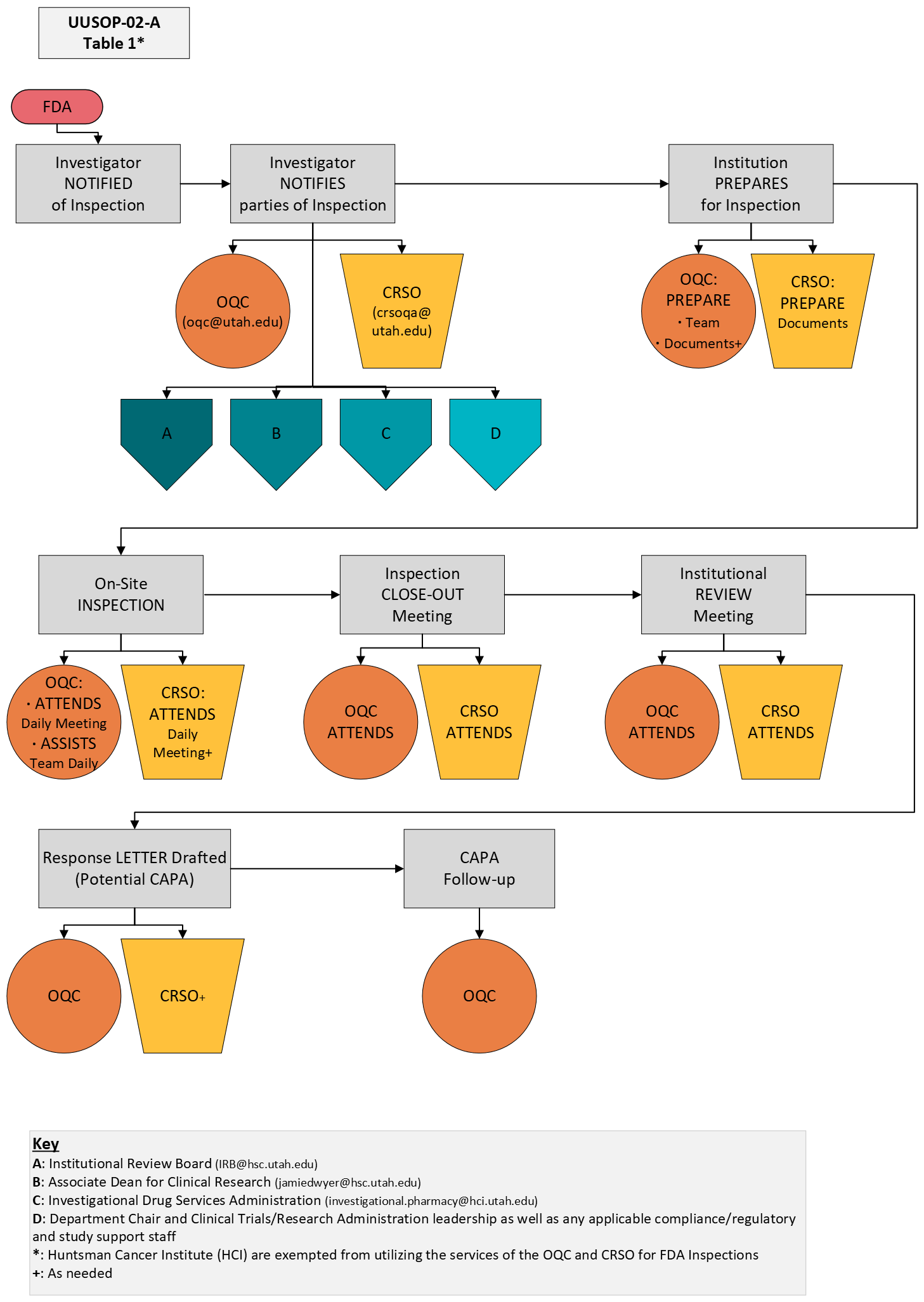
This document serves as a supplement to University of Utah SOP# 002: FDA Inspections. Table 1 below demonstrates the central offices that will need to be notified and involved in the inspection. As noted in the diagram below, specific involvement from the Office of Quality Compliance (OQC) and the Clinical Research Support Office (CRSO) will be required.
Introduction and Purpose
The process for the preparation, conduct, and response to an FDA inspection of a clinical trial is detailed in UUSOP-02: FDA Inspections. In the event a study team is notified of an FDA inspection, or the study team is made aware there is a likelihood or has reason to believe there will be an inspection by the FDA, it is imperative that selected University offices are immediately notified.
Involvement of University central offices will promote success and consistency during FDA inspections. Table 1 below intends to provide additional guidance on which central offices will be involved during each phase of the inspection.
Definitions and Acronyms
Clinical Trial: Clinical trials are clinical research studies involving human participants assigned to an intervention in which the study is designed to evaluate the effect(s) of the intervention on the participant and the effect being evaluated is a health-related biomedical or behavioral outcome.
| CAPA: | Corrective and Preventative Action |
| CRSO: | Clinical Research Support Office |
| FDA: | United States Food and Drug Administration |
| IRB: | Institutional Review Board |
| OQC: | Office of Quality Compliance |
| SOP: | Standard Operating Procedure |
References
- UUSOP-02 FDA Inspections
- UUSOP-07-A Deviations: Documentation and Reporting- Corrective and Preventative Action (CAPA) Plans
|
Version Date |
Change Summary |
|
28Feb2024 |
Original Version |
|
13Aug2025 |
Adding UUSOP-07 Supplement A to references |
